2 Which Set of Coefficients Would Balance the Following Equation
Solving the system along this axis greatly simplifies the mathematics. The following points help us in clearly summarizing the concepts involved in linear equations in one variable.
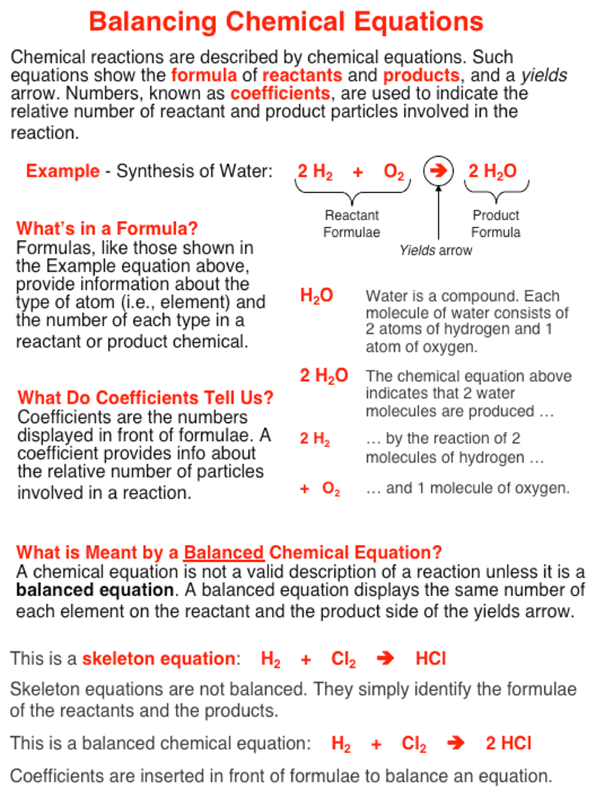
Balancing Chemical Equations Help
3 To get the second equation of motion for this system sum the forces perpendicular to the pendulum.

. Here we take the symmetry axis of the parabola. And some of the examples of non-linear equations are the equation of a circle - x 2 y 2 25 equation of a elipse - x 2 9 y 2 16 1 equation of a hyperbola - x 2 16 - y 2 25 1. The ratio set up from data in the problem will almost always be the one with an unknown in it.
Four molecules NH3 react with six molecules NO to form five molecules N2. 2 Convert grams of the substance given. On the product side c molecules of.
This is likely to make any rounding errors in the number representations very significant and may lead to issues with accuracy of the solution. Start with the first step at the top of the list. Find the values of k for which the quadratic equation kxx 2 6 0 has two equal roots.
The equation for Carbon. 2H 2 O 2--- 2H 2 O. An equation is analogous to a weighing scale balance or seesaw.
2 If you substitute this equation into the first equation you get one of the two governing equations for this system. The equation can be expressed in two ways. When two ratios are set equal this is called a proportion and the whole technique creating two ratios setting them equal is called ratio-and-proportion.
Which of the following actions are permitted in balancing a chemical equation. A chemical equation must be balanced. This should make you nervous because the roots of this equation are between 1-20 but there are numbers here that are O19.
Any member ν j of the null space of a ij will serve to balance a chemical equation involving the set of J molecules comprising the system. The standard form of the quadratic equation that is used by the axis of symmetry calculator. 2KClO3 s 2KCls 3O2 g B.
Now a set of equations must be formulated between the reactant and product side in order to balance each element in the reaction. You should get the following equation. Terms in this set 51 Interpret each chemical equation in terms of interacting particles.
4Ks O2 g 2K2Os A. I rounded off some but I made sure to keep more digits. How many grams of hydrogen gas are needed to produce 1050 grams of water given the following unbalanced chemical reaction.
In this example the following equations can be formed. A preferred stoichiometric vector is one for which all of its elements can be converted to integers with no common divisors by multiplication by a suitable constant. H 2 O 2--- H 2 O.
One ratio will come from the coefficients of the balanced equation and the other will be constructed from the problem. Each side of the equation corresponds to one side of the balance. The coefficients are orders of magnitude apart in size.
This equation does not have any coefficients in front of CH 4 and CO 2. 4NH3 g 6NOg 5N2 g 6H2Og C. If the weights on the two sides are equal the scale balances and in analogy the equality that represents the balance is also balanced if not then the lack of balance corresponds to an.
The axis of symmetry equation comes from the following equations. Different quantities can be placed on each side. 1 Balance the chemical equation.
Correctly order the steps necessary to balance a chemical equation. Y ax2 bx c Where c is the constant form and a b are the. HNO_3 aq BaOH_2 s rightarrow BaNO_3_2 aq H_2O l Ethane is burned with 20 excess air.
1050 g 18015 gmol 582848 mol of H 2 O. Find the roots of the quadratic equation 3x 2 5x 2 0 if they exist using the quadratic formula. On the reactant side a molecules of C 6 H 12 O 6 will contain 6a carbon atoms.
How to balance the following equation. Let us explore that. Determine a The balanced equation assuming complete.
Find the roots of 4x 2 3x 5 0 by the method of completing the square. Two formula units KClO3 decompose to form two formula units KCl and three molecules O2. AC 6 H 12 O 6 bO 2 cCO 2 dH 2 O.
This means that the same _____ and _____ of atoms must appear on both sides of the equation.

Introduction To Balancing Chemical Equations Youtube

Finding Missing Coefficients Youtube
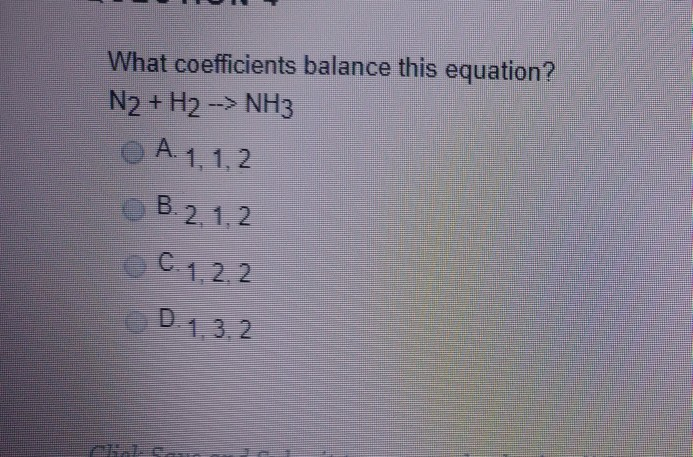
Solved What Coefficients Balance This Equation N2 H2 Chegg Com
No comments for "2 Which Set of Coefficients Would Balance the Following Equation"
Post a Comment